Colour Diagrams: Acid-base Indicators*

Overview
Dear Grade 12 learner
This Mind the Gap study guide helps you to prepare for the end-of-year CAPS Grade 12 exam.
The study guide does NOT cover the entire curriculum, but it does focus on core content of each knowledge area and points out where you can earn easy marks.
You must work your way through this study guide to improve your understanding, identify your areas of weakness and correct your own mistakes.
To ensure a good pass, you should also cover the remaining sections of the curriculum using other textbooks and your class notes.
Overview of the Grade 12 exam
The following topics make up each of the TWO exam papers that you write at the end of the year:
| Cognitive level | Description | Paper 2 (Chemistry) |
| 1 | Remembering/Recall | 15% |
| 2 | Understanding/Comprehension | 40% |
| 3 | Applying and analysing | 35% |
| 4 | Evaluating and creating (synthesis) | 10% |
| Paper | Type of questions | Duration | Total | Date | Marking |
| 2 | Chemistry
| 3 hours | 150 | October/November | External |
| Paper 2: Chemistry Focus | |||||||
| Content | Marks | Total | Duration | Weighting of cognitive levels | |||
| Chemical change | 84 | 150 marks | 3 hours | 15 | 40 | 35 | 10 |
How to use this study guide
This study guide covers selected parts of the different topics of the CAPS Grade 12 curriculum in the order they are usually taught during the year. The selected parts of each topic are presented in the following way:
- An explanation of terms and concepts;
- Worked examples to explain and demonstrate;
- Activities with questions for you to answer; and
- Answers for you to use to check your own work.
The activities are based on exam-type questions. Cover the answers provided and do each activity on your own. Then check your answers. Reward yourself for things you get right. If you get any incorrect answers, make sure you understand where you went wrong before moving on to the next section. In these introduction pages, we will go through the mathematics that you need to know, in particular, algebra and graphs. These are crucial skills that you will need for any subject that makes use of mathematics. Make sure you understand these pages before you go any further.
Top 10 study tips
- Have all your materials ready before you begin studying – pencils, pens, highlighters, paper, etc.
- Be positive. Make sure your brain holds on to the information you are learning by reminding yourself how important it is to remember the work and get the marks.
- Take a walk outside. A change of scenery will stimulate your learning. You’ll be surprised at how much more you take in after being outside in the fresh air.
- Break up your learning sections into manageable parts. Trying to learn too much at one time will only result in a tired, unfocused and anxious brain.
- Keep your study sessions short but effective and reward yourself with short, constructive breaks.
- Teach your concepts to anyone who will listen. It might feel strange at first, but it is definitely worth reading your revision notes aloud.
- Your brain learns well with colours and pictures. Try to use them whenever you can.
- Be confident with the learning areas you know well and focus your brain energy on the sections that you find more difficult to take in.
- Repetition is the key to retaining information you have to learn. Keep going – don’t give up!
- Sleeping at least 8 hours every night, eating properly and drinking plenty of water are all important things you need to do for your brain. Studying for exams is like strenuous exercise, so you must be physically prepared.
Mnemonics
A mnemonic code is a useful technique for learning information that is difficult to remember. Here’s the most useful mnemonic for Mathematics, Mathematical Literacy, and Physical Science:
BODMAS:
B– Brackets
O– Of or Orders: powers, roots, etc.
D– Division
M– Multiplication
A– Addition
S– Subtraction
Throughout the book you will be given other mnemonics to help you remember information. The more creative you are and the more you link your ‘codes’ to familiar things, the more helpful your mnemonics will be.
Mind maps
There are several mind maps included in the Mind the Gaps guides, summarising some of the sections.

Mind maps work because they show information that we have to learn in the same way that our brains ‘see’ information. As you study the mind maps in the guide, add pictures to each of the branches to help you remember the content. You can make your own mind maps as you finish each section.
How to make your own mind maps:
- Turn your paper sideways so your brain has space to spread out in all directions.
- Decide on a name for your mind map that summarises the information you are going to put on it.
- Write the name in the middle and draw a circle, bubble or picture around it.
- Write only key words on your branches, not whole sentences.
- Keep it short and simple.
- Each branch should show a different idea.
- Use a different colour for each idea.
- Connect the information that belongs together.
- This will help build your understanding of the learning areas.
- Have fun adding pictures wherever you can. It does not matter if you can’t draw well.
On the day of the exam
- Make sure you have all the necessary stationery for your exam, i.e. pens, pencils, eraser, protractor, compass, calculator (with new batteries). Make sure you bring your ID document and examination admission letter.
- Arrive on time, at least one hour before the start of the exam.
- Go to the toilet before entering the exam room. You don’t want to waste valuable time going to the toilet during the exam.
- Use the 10 minutes reading time to read the instructions carefully. This helps to ‘open’ the information in your brain. Start with the question you think is the easiest to get the flow going.
- Break the questions down to make sure you understand what is being asked. If you don’t answer the question properly you won’t get any marks for it. Look for the key words in the question to know how to answer it. Lists of difficult words (vocabulary) is given a bit later on in this introduction.
- Try all the questions. Each question has some easy marks in it so make sure that you do all the questions in the exam.
- Never panic, even if the question seems difficult at first. It will be linked with something you have covered. Find the connection.
- Manage your time properly. Don’t waste time on questions you are unsure of. Move on and come back if time allows. Do the questions that you know the answers for, first.
- Write big and bold and clearly. You will get more marks if the marker can read your answer clearly.
- Check weighting – how many marks have been allocated for your answer? Take note of the ticks in this study guide as examples of marks allocated. Do not give more or less information than is required.
Question words to help you answer questions
It is important to look for the question words (the words that tell you what to do) to correctly understand what the examiner is asking. Use the words in the table below as a guide when answering questions.
| Question word | What is required of you |
| Analyse | Separate, examine and interpret |
| Calculate | This means a numerical answer is required – in general, you should show your working, especially where two or more steps are involved |
| Classify | Group things based on common characteristics |
| Compare | Point out or show both similarities and differences between things, concepts or phenomena |
| Define | Give a clear meaning |
| Describe | State in words (using diagrams where appropriate) the main points of a structure/process/phenomenon/ investigation |
| Determine | To calculate something, or to discover the answer by examining evidence |
| Differentiate | Use differences to qualify categories |
| Discuss | Consider all information and reach a conclusion |
| Explain | Make clear; interpret and spell out |
| Identify | Name the essential characteristics PAY SPECIAL ATTENTION |
| Label | Identify on a diagram or drawing |
| List | Write a list of items, with no additional detail |
| Mention | Refer to relevant points |
| Name | Give the name (proper noun) of something |
| State | Write down information without discussion |
| Suggest | Offer an explanation or a solution |
| Tabulate | Draw a table and indicate the answers as direct pairs |
Vocabulary
The following vocabulary consists of all the difficult words used in Mind the Gap Mathematics, Mathematical Literacy, and Physical Science. We suggest that you read over the list below a few times and make sure that you understand each term. Tick next to each term once you understand it so you can see easily where the gaps are in your knowledge.
| approximate | (v. & adj.). Come close to (v); roughly, almost, not perfectly accurate, close but not exact. The verb is pronounced “approxi-mayt” and the adjective is pronounced “approxi-mitt”. |
| aquatic | (adj). Growing or living near or in water. |
| arbitrary | (adj). Based on random choice; unrestrained and autocratic. |
| C | |
| category | (n). Class or group of things. |
| cause | (v). Make something happen. |
| cause | (n). The person or thing that makes something happen; an aim or movement to which a person is committed. |
| causality | (n). Someone or something responsible for a result. |
| collide | (v). To crash into; to hit. |
| complex | (adj). Consisting of many different parts; not easy to understand (n) a group or system of things connected in a complicated way. |
| component | (n). A part. |
| compose | (v). To make up from parts. |
| composite | (n). Something made up of parts; (adj) made up of several parts. |
| condition | (n). The state something is in; the situation that must exist before something else is possible. |
| conjunction | (n). When two or more things come together at the same point; in grammar, a part of speech that connects words, sentences, phrases or clauses, e.g.: “and” |
| consider | (v). Think about. |
| contrast | (v). Show the difference between; |
| (n). something that is very different from what it is being compared with. | |
| conversely | (adv). The opposite of. |
| counteract | (v). Act against something in order to stop it. |
| D | |
| data (pl), datum (sing) | (n). Information given or found. |
| deduce | (v). To work something out by reasoning. |
| deduction | (n). Conclusion or idea that someone has worked out. |
| define | (v). Give the meaning of a word or words. |
| definition | (n). The meaning of a word or words. |
| deliver | (v). To bring and hand over. |
| denote | (v). To refer to or mean something. |
| determine | (v). Work out, usually by experiment or calculation. |
| discreet | (adj). Careful, polite. |
| discrete | (adj). Single, separate, distinct, a part. |
| E | |
| effect | (n). Result. |
| effect | (v). Carry out, do, enact. |
| eject | (v). Force or throw something or someone out violently or suddenly. |
| elapse | (v). Pass by or finish, e.g., time. |
| establish | (v). Show or prove, set up or create. |
| exceed | (v). Go beyond. |
| excess | (n). More than necessary. |
| excluding | (prep). Not including. |
| exclusive | (adj). Excluding or not admitting other things; reserved for one particular group or person. |
| exemplar | (n). A good or typical example. |
| exempt | (v). To free from a duty. |
| exempt | (adj). Be freed from a duty. |
| exemption | (n). Being freed from an obligation. |
| exhibit | (v). To show or display. |
| exhibit | (n). A part of an exhibition. |
| expel | (v). Force someone or something to leave a place. Eject. |
| extent | (n). The area covered by something. |
| F | |
| factor | (n). A circumstance, fact or influence that contributes to a result; a component or part. |
| factory | (n). A place where goods are made or put together from parts. |
| find | (v). Discover or locate. |
| find | (n). Results of a search or discovery. |
| finding | (n). Information discovered as the result of an inquiry. |
| fixed | (adj). Not able to move, attached; or repaired, not broken. |
| format | (n). Layout or pattern; the way something is laid out. |
| G | |
| global | (adj). Found all over the world (globe). |
| H | |
| hazard | (n). Something dangerous. |
| heterogeneous | (adj). Made up of many different parts. |
| homogeneous | (adj). Uniform, made up of the same types of parts. |
| hypothesis | (n). A theory or proposed explanation. |
| hypothetical | (adj). Theoretical or tentative; waiting for further evidence. |
| I | |
| identify | (v). Recognise or point out. |
| illustrate | (v). Give an example to show what is meant; draw. |
| impair | (v). Weaken or damage. |
| imply | (v). Suggest without directly saying what is meant. |
| indicate | (v). Point out or show. |
| initial | (n). First. |
| initiation | (n). The action of beginning something; the action of admitting somebody into a group or organisation. |
| insufficient | (adj). Not enough. |
| interchange- | (adj). Can be swapped or |
| able | exchanged for each other. |
| investigate | (v). Carry out research or a study. |
| issues | (v). Comes out of. |
| issues | (n). An important problem or a topic for debate. |
| M | |
| macroscopic | (adj). Visible without being made bigger. |
| manipulate | (v). Handle or control (a thing or a person). |
| microscopic | (adj). Very small, not visible without being made bigger. |
| motivate | (v). Give someone a reason for doing something. |
| multiple | (adj). Many. |
| N | |
| negligible | (adj). Small and insignificant; can be ignored. From “neglect” (ignore). |
| numerical | (adj). Relating to or expressed as a number or numbers. |
| numerous | (adj). Many. |
| O | |
| observe | (v). Look at; watch carefully. |
| obtain | (v). Get. |
| occur | (v). Happen. |
| operate | (v). Work; drive; control. |
| optimal | (adj). Best; most favourable. |
| optimum | (adj). Best; (n) the most |
| favourable situation for growth or success. | |
| overabundance | (n). More than enough; too much. |
| P | |
| phenomenon | (n). A fact or situation that is seen to exist or happen. |
| phenomena | (n). Plural of phenomenon. |
| prefix | (n). Part of a word that is attached to the beginning of many different words, changing their meaning, e.g., prehistoric – before written records were kept. |
| prepare | (v). Make ready before an event; set things up. |
| principal | (n). Head of a school. |
| principal | (adj). Main or most important. |
| principle | (n). A basic truth that guides the way a person behaves. |
| provide | (v). Make available for use; supply. |
| Q | |
| quality | (n). The standard of something compared to other similar things; a characteristic of someone or something. |
| R | |
| reciprocal | (adj). Given or done in return. |
| record | (v). Make a note of something in order to refer to it later (pronounced ree-cord). |
| record | (n). A note made in order to refer to it later; evidence of something; a copy of something (pronounced rec-cord. |
| relative | (adj). Considered in relation to something else; compared to. |
| relative | (n). A family member. |
| represent | (v). Be appointed to act or speak for someone; amount to. |
| resolve | (v). Finalise something or make it clear; bring something to a conclusion. |
| respect | (v). Admire something or someone; consider the needs or feelings of another person. |
| respectively | (adj). In regards to each other, in relation to items listed in the same order. |
| S | |
| simultaneously | (adv). At the same time. |
| site | (n). Place. |
| suffice | (v). Be enough. |
| surplus | (adj). More than is needed. |
| survey | (n). A general view, examination, or description of someone or something. |
| survey | (v). Look closely at or examine; consider a wide range of opinions or options. |
| T | |
| tendency | (n). An inclination to do something in a particular way; a habit. |
| tertiary | (adj). Third level. |
| U | |
| uniform | (n). Standardised clothing. |
| uniform | (adj). Remaining all the same at all times; unchanging. |
| V | |
| verify | (v). Show to be true; check for truth; confirm. |
| vice versa | (adv). The other way round. |
| versus | (prep). Against. Abbreviated “vs” and sometimes “v”. |
Technical terms
| A | |
| absorption | (n). To take into; the process of |
| taking something in. | |
| account | (n. & v.). Finance: A record of |
| income and expenditure. To | |
| explain (v), e.g. “Account for why | |
| the sky is blue”. | |
| acetic | (adj). Pertaining to vinegar; an |
| organic molecule containing two | |
| carbons. See organic, eth- for | |
| more. | |
| acetone | (n). Propanone. CH3COCH3; the |
| ketone of acetic acid. See ketone | |
| for more. | |
| acetylene | (n). Ethyne, C2H2. Used in welding |
| torches (blowtorches). See also | |
| alkene. | |
| acid | (n). A proton donor or substance |
| that ionises into H+ or H3O+ when | |
| dissolved in water; sour-tasting | |
| substance; corrosive; pH below 7. | |
| See also base and alkali. | |
| acidified | (adj). To have been made acidic. |
| acidity | (n). How acid something is. |
| activated | (adj). Made to function. Chemistry: |
| something moved into an unstable | |
| higher-energy level or state. | |
| Usually “activated complex”, the | |
| combination of reactants just | |
| before they turn into products. See | |
| also reactant, reagent, product. | |
| activation | (n). The process of activating |
| something. “Activation energy”, | |
| the required energy to create an | |
| activated complex. | |
| aerosol | (n). A solution of substances in air or |
| other gas, e.g. as in an aerosol can. |
| affinity | (n). A liking for something; |
| an attraction to something; a | |
| tendency to react with something | |
| (chemistry). See also paraffin. | |
| alcohol | (n). In common usage, ethanol |
| C2H5OH. Technically, any organic | |
| substance or molecule containing | |
| an -OH group. See organic. | |
| aldehyde | (n). Any organic molecule containing |
| -CHO, formed by oxidising alcohols. | |
| See alcohol and organic. | |
| algae | (n). Adjective: algal (pertaining to |
| algae); an aquatic plant (lives in | |
| water), which lacks leaves, stems, | |
| roots. | |
| algebra | (n). A mathematical system |
| where unknown quantities are | |
| represented by letters, which | |
| can be used to perform complex | |
| calculations through certain rules. | |
| alkali | (n). See base. |
| alkane | (n). An organic molecule or |
| compound or substance which | |
| contains only single bonds | |
| between carbons. See organic. | |
| alkene | (n). An unsaturated organic |
| molecule, compound or substance, | |
| which contains at least one double | |
| bond between carbons. See | |
| organic and unsaturated. | |
| alkyl | (n). A prefix (word part) which shows |
| that the word after it has an alkane | |
| group attached to it, by removing | |
| one hydrogen from the alkane. | |
| alkyne | (n). An unsaturated organic |
| molecule, compound or substance, | |
| which contains at least one triple | |
| bond between carbons. See | |
| organic and unsaturated. | |
| amalgam | (n). General use: a mixture. In |
| chemistry, specifically a mercury | |
| alloy. | |
| amide | (n). An organic compound |
| containing the group -C(O)NH2; an | |
| inorganic compound containing | |
| the group NH2–. | |
| amine | (n). The same as an amide except |
| the -NH2 can be attached to | |
| anything, and does not have the | |
| CO group shown above. | |
| ammonia | (n). NH3. |
| ammonium | (adj). NH4+, found as a cation or as |
| part of a salt. See salt and cation. | |
| amphiprotic | (n). See ampholyte. |
| ampholyte | (n). A substance that can act as an |
| acid or base. See acid, base. |
| anions | (n). A negative ion. See cation, ion. |
| anode | (n). The negative electrode of |
| a cell or current supplier; the | |
| positive electrode of an electrolytic | |
| system; attracts negative ions. See | |
| electrode, cathode. | |
| antacid | (n). A substance used to neutralise |
| (react with) acid. E.g. chalk. See | |
| acid, neutralise. | |
| apparatus | (n). Equipment; parts of a scientific experiment. |
| aqueous | (n). Dissolved in water. |
| Arrhenius | (n). Arrhenius’ theory of acids and bases: That acids produce H+ or H3O+ in water, and bases produce OH–. See Brønsted-Lowry. |
| asbestos | (n). A fireproof fibrous substance containing silicon used for fireproofing. |
| asbestosis | (n). A lung disease caused by inhaling asbestos fibres, can lead to cancer. |
| atm | (n). Abbreviation: atmospheres of pressure (1 atm = 101,3 kPa). The pressure of the air at sea level. Same as “bar” (barometric pressure). |
| ATM | (n). Abbreviation: automatic teller machine. |
| atmosphere | (n). The air or the gases surrounding a planet; the sky; as a unit of measurement, see atm. |
| atmospheric | (adj). To do with the atmosphere. |
| atom | (n). The smallest unit of a chemical element, which, if broken down further, no longer behaves in the same way chemically. Consists of a nucleus or centre part which is positively charged, and an electron cloud (negatively charged) which surrounds the nucleus. See nuclear. |
| attract | (v). To bring something closer. |
| average | (n). Mathematics: The sum of parts divided by the quantity of parts. In common use: neither very good, strong, etc., but also neither very weak, bad, etc; the middle. In Physical Science and Mathematics: if you are asked to find the average, you always have to calculate it using the information you have. For example, the average of (1;2;3) is 2, because (1+2+3)/3 = 2. See also mean, median and mode. |
| avogadro (constant or number) | (n). 6,023 × 1023 particles; one mole. See mole and mol. |
| axis (sing), axes (pl, pronounced “akseez”) | (n). A line along which points can be plotted (placed), showing how far they are from a central point, called the origin. See origin. “Vertical axis” or “y-axis” refers to how high up a point is above the origin (or how far below). “Horizontal axis” or “x-axis” refers to how far left or right a point is away from the origin. |
| B | |
| bakelite | (n). A type of hard, brittle plastic |
| that can’t melt once it has set | |
| or taken shape (thermosetting), | |
| made from phenol C6H5OH, and | |
| formaldehyde (methanal), CH2O. | |
| balance | (v. & n.). To make two things equal |
| (v); a scale to weigh objects (n). | |
| Chemistry: to compare two sides | |
| of a chemical equation and make | |
| sure that there are the same | |
| numbers of atoms on both sides. | |
| base | (n). A proton acceptor, or substance |
| that ionises into OH– when | |
| dissolved in water; a bitter-tasting | |
| substance, corrosive, pH above 7. | |
| See also acid. Do not confuse with | |
| common everyday use, meaning | |
| “the bottom” or “low”. | |
| basic | (n). Bitter or made of a base. Do not |
| confuse with popular use, meaning | |
| “low” or “simple” or “crude”. | |
| battery | (n). A collection of cells connected |
| in series (end-to-end). See cell. In | |
| common use, “battery” is used to | |
| mean the same as “cell” (e.g. a | |
| penlight or AA cell), but this use is | |
| incorrect except for a car battery, | |
| which consists of a series of cells. | |
| benzoic | (adj). Contains benzene or a |
| benzene ring. | |
| bi- | (prefix). Two. |
| bicarbonate | (n). Any salt containing the ion |
| -HCO –. So called because the | |
| 3 | |
| carbonate (CO32–) attaches to | |
| another ion and the hydrogen | |
| (two bonds). The name “hydrogen | |
| carbonate” is now preferred. | |
| biodegradable | (adj). Can be broken down by |
| natural processes e.g. involving | |
| bacteria, moulds, fungus, etc. | |
| biodiesel | (n). Diesel (a type of petrol) made |
| from plants rather than fossil fuels | |
| (coal, oil). |
| BODMAS | (abbr). Brackets, of/orders |
| (powers, squares, etc), division, | |
| multiplication, addition, subtraction. | |
| A mnemonic (reminder) of the | |
| correct order in which to do | |
| mathematical operations. | |
| boil | (v). Physics: to cause a liquid’s |
| vapour pressure to exceed | |
| the pressure of the gas in the | |
| container, usually by heating it, | |
| but it can be done by lowering | |
| the pressure of the gas in the | |
| container, too. See vapour | |
| pressure. In common usage, to | |
| make a liquid hot until it bubbles. | |
| bond | (n). A connection. In physics and |
| chemistry, between atoms and | |
| molecules. | |
| breadth | (n). How wide something is. From |
| the word “broad”. | |
| brine | (n). A saturated salt solution (a |
| mixture of water and salt which | |
| can’t dissolve any more salt). | |
| bromide | (n). Something containing bromine, |
| usually one ion. See ion. | |
| bromo- | (prefix). Something containing |
| bromine. | |
| bromothymol | (n). A type of acid-base indicator |
| (blue) | used to tell whether something |
| is an acid or base. Turns blue (in | |
| base) or yellow (in acid). | |
| Brønsted-Lowry | (n). A theory of acids and bases |
| which says that acids are proton | |
| donors (they give away protons), | |
| and bases are proton acceptors | |
| (they take protons). Since H+ is | |
| just a proton, this does not mean | |
| something different from the | |
| Arrhenius theory that an acid is a | |
| substance that dissolves into H+ in | |
| water. See proton, Arrhenius. | |
| but- | (prefix). Four carbons. Pronounced |
| “beaut”. E.g. butane is a four- | |
| carbon alkane. | |
| C | |
| calibrate | (v). To adjust a measuring tool |
| or measurement against a | |
| known accurate measurement to | |
| ensure that the measuring tool or | |
| measurement is accurate; to check | |
| a measurement or measuring | |
| tool’s accuracy; to mark with | |
| accurate measurements using a | |
| standard scale like cm, mm, mℓ, | |
| etc. Common use: to assess or | |
| evaluate carefully. |
| carbohydrate | (n). Organic compounds containing |
| carbon and hydrogen, occurring | |
| in foods and living tissues and | |
| including sugars, starch, and | |
| cellulose. They contain hydrogen | |
| and oxygen in the same ratio | |
| as water (2:1). Not the same | |
| as hydrocarbons, which are any | |
| substances containing mostly | |
| hydrogen and carbon. | |
| carbonate | (n). -CO32– |
| carbonic | (adj). Anything containing carbon, |
| or more specifically, CO2 | |
| carbonyl | (adj). Containing double bonded |
| carbon and oxygen: =C=O. | |
| carboxyl | (adj). Containing -COOH. |
| carboxylic | (adj). Carboxyl-containing. |
| Cartesian | (adj). Anything believed or |
| proposed by Rene Descartes. | |
| In particular, the x-and-y axis | |
| coordinate system. | |
| catalyst | (n). A substance that alters the |
| rate of a chemical reaction without | |
| itself being consumed in the | |
| reaction. Without qualification, or | |
| as “positive catalyst”, something | |
| that starts or speeds up a reaction. | |
| A “negative” catalyst slows down a | |
| reaction. | |
| catalytic | (n). Containing or using a catalyst. |
| cathode | (n). The positive electrode of a cell |
| or current supplier; the negative | |
| electrode of an electrolytic | |
| system; attracts positive ions. See | |
| electrode, anode, ion. | |
| cathodic | (adj). Involving a cathode. Cathodic |
| protection: To use a more reactive | |
| metal to protect a less reactive | |
| metal from oxidation. See anode, | |
| cathode, oxidise. | |
| cation | (n). A positively charged ion. See |
| anion, ion. | |
| caustic | (adj). Basic; a base. |
| cell | (n). An apparatus that generates |
| electricity using electrochemistry. | |
| An AA or Penlight battery, as it is | |
| commonly called, is a cell. A car | |
| battery consists of a number of | |
| cells inside a single container. | |
| CFC | (n). A chlorofluorocarbon. A |
| substance containing carbon, | |
| chlorine and fluorine. Responsible | |
| for breaking down ozone (O3) which | |
| protects us from too much UV | |
| radiation from the sun. |
| chain | (n). Chemistry: a long series of |
| atoms bonded together, usually | |
| carbon. | |
| charge | (n). Chemistry: having too |
| many or too few electrons | |
| (most commonly), resulting in | |
| a substance ionising. A positive | |
| charge results from too few | |
| electrons, and a negative charge | |
| from too many electrons. Physics: | |
| a basic feature of all physical | |
| electromagnetic particles, except, | |
| e.g. neutrons and photons, which | |
| have zero charge. All protons have | |
| a positive charge, all electrons | |
| have a negative charge. | |
| chart | (v). To draw a diagram comparing |
| values on Cartesian axes. | |
| Le Châtelier’s | (n). That in reversible reactions, |
| Principle | chemical systems will favour the |
| forward or reverse reaction to | |
| minimise the change imposed | |
| on the system. If a chemical | |
| equilibrium is disturbed by | |
| changing the conditions, the | |
| position of equilibrium moves to | |
| counteract the change. | |
| chloro- | (prefix). Containing chlorine. |
| chlorofluoro- | See CFC. |
| carbons | |
| chloroform | (n). CHCℓ2. A liquid formerly used |
| as an anaesthetic. | |
| chlorophyll | (n). A green substance found |
| in plants which enables | |
| photosynthesis (broadly, | |
| generating food from CO2). See | |
| photosynthesis. | |
| coefficient | (n). A constant value placed |
| next to an algebraic symbol as a | |
| multiplier. Same as constant (see | |
| below). Or: a multiplier or factor | |
| that measures a property, e.g. | |
| coefficient of friction. | |
| combustion | (n). The process of burning, usually |
| in oxygen. Rapid oxidation. | |
| completion | (n). Chemistry: when a reaction |
| no longer proceeds (continues) | |
| because it has run out of one | |
| or more of the reactants. See | |
| reactant, reaction. | |
| complex | (n). See activated. |
| (activated) |
| compound | (n). A substance made up of |
| molecules consisting of more | |
| than one different type of atom, | |
| chemically bonded in a constant | |
| ratio. E.g. Water (H2O) is a | |
| compound, but Sulphur powder | |
| (S) is not. In a compound, the | |
| original chemicals (reactants) have | |
| reacted or merged to form a new | |
| substance. Compare to mixture. | |
| compressed | (adj). Subjected to pressure, |
| squashed. | |
| concentration | (n). The number of moles of |
| substance per unit volume. | |
| See mol, moles. How “strong” a | |
| solution is. See solution. | |
| condensation | (n). When a vapour or gas cools |
| down and starts to collect into | |
| larger droplets; changing phase | |
| from vapour or gas to liquid. | |
| Condensation reaction: to produce | |
| a larger molecule from two smaller | |
| ones. | |
| conditions | (n). Physics and Chemistry: how |
| (STP) | the environment is: temperature |
| and pressure. STP (Standard | |
| Temperature and Pressure is 25°C | |
| and 1 atm). | |
| conjugate | (n). To join together. Chemistry: two |
| things that belong together, e.g. | |
| conjugate acid-base pairs. | |
| conservation | (n). A law which describes |
| something that does not change. | |
| E.g. the conservation of matter- | |
| energy says that matter-energy | |
| cannot be created or destroyed, | |
| only transformed from one form | |
| into another. There are a number | |
| of other conservations, e.g. | |
| momentum and torque. | |
| constant | (n). See coefficient. Means |
| “unchanging”. | |
| contaminate | (v). Chemistry: to introduce |
| impurities or other substances | |
| which are not meant to be part of | |
| a reaction. | |
| control | (n. and v.). To ensure something |
| does not change without | |
| being allowed to do so (v); | |
| an experimental situation to | |
| which nothing is done, in order | |
| to compare to a separate | |
| experimental situation, called the | |
| ‘experiment’, in which a change | |
| is attempted. The control is then | |
| compared to the experiment to see | |
| if a change happened. |
| control variable | (n). A variable that is held constant |
| in order to discover the relationship | |
| between two other variables. | |
| “Control variable” must not be | |
| confused with “controlled variable” | |
| (see independent variable). | |
| coordinate | (n). The x or y location of a point on |
| a Cartesian graph, given as an x or | |
| y value. Coordinates (pl) are given | |
| as an ordered pair (x, y). | |
| correlate | (v). To see or observe a |
| relationship between two things, | |
| without showing that one causes | |
| the other. | |
| correlation | (n). That there is a relationship |
| between two things, without | |
| showing that one causes the other. | |
| correspond | (v). To pair things off in a |
| correlational relationship. For two | |
| things to agree or match. E.g. A | |
| corresponds to 1, B corresponds to | |
| 2, C corresponds to 3, etc. | |
| corrode | (v). Chemistry: to destroy by |
| gradual chemical action. Usually | |
| refers to acidic action. Compare | |
| to erode. General use: to destroy | |
| gradually. | |
| corroding | (adj). A process or substance |
| that corrodes; to be subject to | |
| corrosion. | |
| corrosion | (n). To corrode. |
| corrosive | (adj). To be capable of corroding |
| something. | |
| counteract | (v). Oppose or resist. |
| covalent | (adj). Chemistry: a bond which |
| results from sharing electrons | |
| between atoms. Compare ionic | |
| bond. | |
| cracking | (n). A process of breaking a |
| complex organic molecule into | |
| simpler parts using heat and | |
| pressure. | |
| cubed | (adj). The power of three; |
| multiplied by itself three times. | |
| cubic | (adj). Shaped like a cube; having |
| been multiplied by itself three | |
| times. | |
| current | (n). Flowing electrons. |
| D | |
| decompose | (v). To break down into |
| components. |
| degradable | (adj). Capable of breaking down or |
| being broken down. | |
| dehydrating | (adj). To remove water from. A |
| (agent) | “dehydrating agent” is a substance |
| which can remove water from | |
| another substance. E.g. H2SO4, | |
| ethanol. | |
| dehydration | (n). The process of removing water |
| from a substance. | |
| dehydrogena- | (n). The process of removing |
| tion | hydrogen from a substance. |
| dehydrohalo- | (n). To remove hydrogen and a |
| genation | halogen from a substance. See |
| halogen. | |
| denominator | (n). See divisor. In popular speech: |
| a common factor. | |
| depend | (v). To be controlled or determined |
| by something; to require something | |
| to happen or exist first. | |
| dependent | (adj/n). A variable whose value |
| (variable) | depends on another; the thing |
| that comes out of an experiment, | |
| the effect; the results. See also | |
| independent variable and control | |
| variable. The dependent variable | |
| has values that depend on the | |
| independent variable, and we plot | |
| it on the vertical axis. | |
| depleted | (adj). Having been used up; run |
| out of. | |
| deposit | (n). Finance: to place money into |
| an account. Physical Science: to | |
| cover a surface of one substance | |
| with another substance, e.g. metal | |
| plating on an electrode. | |
| determine(s) | (v). To cause; to ensure that; to set |
| (causation) | up so that; to find out the cause of. |
| di- | (prefix). Two. |
| diamine | (n). A substance containing two |
| amine groups. See amine. | |
| diammonium | (n). Having two ammonium (NH4) |
| groups. See ammonium. | |
| diaphragm | (n). A thin sheet of any substance |
| covering a gap. Biology: the muscle | |
| below the lungs which moves to | |
| cause breathing. Electrochemistry: | |
| a thin sheet inside a cell which | |
| separates the electrodes. It is | |
| porous and allows solutions | |
| containing ions through, but serves | |
| to separate gas products. | |
| difference | (n). Mathematics: subtraction. |
| Informally: a dissimilarity. How | |
| things are not the same. |
| dilute | (adj. & v). To lower the |
| concentration of a solution. See | |
| solution and concentration (v): | |
| a solution which has had its | |
| concentration lowered (adj). | |
| dilution | (n). The opposite of concentration; |
| how low a concentration is, | |
| measured in mol/dm3; the process | |
| of diluting. | |
| dimer | (n). A molecule made of two |
| identical parts. See also polymer. | |
| diode | (n). A semiconductor device with |
| two terminals (electrodes), usually | |
| allowing current to flow in one | |
| direction only. | |
| diol | (n). A molecule with two alcohol/ |
| hydroxyl (OH) groups. | |
| dipole | (n). A polarised molecule with a |
| distinctly positive and distinctly | |
| negatively-charged end. | |
| diprotic | (n). Having two protons. |
| displace | (v). To move or relocate something. |
| dissipate | (v). To disperse or scatter (e.g. |
| gas). Thermodynamics (Energy): | |
| to cause energy to be lost as heat. | |
| Popular use: to disappear. | |
| dissociate | (v). To break apart; to no longer be |
| associated with. | |
| dissolve | (v). To break up into ions within a |
| solution (usually water); to mix a | |
| solid (usually powder) into a liquid, | |
| to form a solution. See solution, | |
| ion. Alternative popular use: to | |
| bring to an end. | |
| distil(l) | (v). To purify through repeated |
| heating of a liquid and collection | |
| of condensation. The heating | |
| process causes the liquid to form | |
| gas or vapour, which condenses | |
| on the side of the heating vessel | |
| (container) or an exit tube, leaving | |
| impurities behind in the heating | |
| vessel. | |
| distribution | (n). How something is spread |
| out. Mathematics: the range and | |
| variety of numbers as shown on a | |
| graph. | |
| disturb | (v). Chemistry: to mix or stir a liquid |
| or solution; to shake it up. | |
| divisor | (n). The number below the line |
| in a fraction; the number that is | |
| dividing the other number above | |
| the fraction line. See numerator, | |
| denominator. |
| domain | (n). The possible range of x-values |
| for a graph of a function. See | |
| range. | |
| durable | (adj). Tough; something that can |
| endure. | |
| dynamic | (adj). Changing often. Relating |
| to forces that produce motion. | |
| Opposite of static. See static and | |
| electrostatic. | |
| E | |
| ecosystem | (n). An integrated, complex, |
| interacting, mutually dependent | |
| living system or environment. | |
| electric | (adj). Containing electricity |
| (electrons). | |
| electro- | (adj). Where chemical reactions |
| chemical | cause the release of electrons, |
| usually into a circuit. General use: | |
| anything relating to electrical and | |
| chemical phenomena. | |
| electrode | (n). General use: the point where |
| electrons enter or exit a power | |
| source or a circuit. Specifically | |
| (Electrochemistry): Part of a circuit | |
| dipped into a solution to receive or | |
| release electrons. See anode and | |
| cathode. | |
| electrolysis | (n). The splitting of a chemical |
| into ions. (The chemical is usually | |
| dissolved in water or another | |
| solution.) It is done by means of | |
| electricity. See electrochemical. | |
| electrolyte | (n). A substance (usually liquid |
| or gel solution) which contains | |
| a compound that will be split by | |
| electricity. Ionisable solutions or | |
| components. | |
| electromotive | (adj). Usually electromotive force |
| or emf. The potential difference | |
| caused by electromagnetism, | |
| which causes current to flow. | |
| Producing a current with | |
| electromagnetism. See emf. | |
| electron | (n). A fundamental physical |
| particle bearing a negative charge, | |
| weighing approximately 9 × 10−28g, | |
| which is found around atomic | |
| nuclei in areas called ‘orbitals’. | |
| Responsible for electricity and | |
| chemical reactions. Symbol e–. See | |
| proton, nucleus. |
| electroplate | (v). To cover a surface of a less |
| valuable substance with a more | |
| valuable metal, using electrolysis. | |
| element | (n). Mathematics: part of a set |
| of numbers. Physics: a pure | |
| substance made only of atoms of | |
| one type, with the same number | |
| of protons in each nucleus. An | |
| element cannot be broken down | |
| further without losing its chemical | |
| properties. Each element has a | |
| unique atomic number which is the | |
| number of protons in the nucleus. | |
| See nucleus, atom, isotope. | |
| Popular use: part of. | |
| eliminate | (v). To remove or get rid of. |
| Mathematics: to cancel a factor | |
| out of one side of an equation by | |
| dividing by that factor throughout, | |
| or by substituting in another | |
| formula or value that is equal. | |
| Chemistry: to produce a smaller | |
| substance as a by-product | |
| from reacting more complex | |
| substances, usually water or CO2; | |
| in the sense of: to remove those | |
| molecules from the reaction. | |
| emf | (abbr). Same as electromotive |
| force. Always written in lowercase | |
| (small letters). | |
| emission | (n). Something released, e.g. gas, |
| light, heat. | |
| emit | (v). To release. |
| empirical | (adj). Relating to the senses or to |
| things that you can see, touch, | |
| taste, etc. Chemistry: empirical | |
| formula: a formula giving the | |
| proportions of the elements | |
| present in a compound but not the | |
| actual numbers or arrangement | |
| of atoms; the lowest ratio of | |
| elements without giving structure | |
| or quantities. | |
| emulsion | (n). Small particles or droplets of |
| a substance or liquid which do | |
| not dissolve in a different liquid; | |
| suspended or floating within that | |
| liquid, e.g. to mix oil and water by | |
| shaking them up. | |
| endothermic | (adj). Taking in heat, ΔH > 0. See |
| enthalpy, exothermic. | |
| energetic | (adj). Having a lot of energy; |
| performing a lot of work. |
| energy | (n). Work or the ability to do |
| work. There are various forms of | |
| energy: motion (Ek), light energy | |
| (photons), electrical energy, heat, | |
| etc. Energy can neither be created | |
| nor destroyed, but only converted | |
| from one form to another. See | |
| conservation. | |
| enthalpy | (n). The total heat content of a |
| system, H, including the chemical | |
| energy. | |
| equilibria (pl), | (n). The state of being in balance. |
| equilibrium | Chemistry: when the forward |
| (sing) | reaction rate is equal to the |
| reverse reaction rate. See Le | |
| Châtelier’s Principle. | |
| erode | (v). To wear away by means of |
| friction (rubbing). | |
| ester | (n). An organic compound |
| produced by bonding an alcohol | |
| to a carboxylic (organic) acid, | |
| by means of dehydration. See | |
| carboxylic, organic, alcohol, | |
| dehydration. Responsible for fruit | |
| flavours and many pleasant odours | |
| (smells). | |
| esterfication | (n). The production of esters. |
| estimate | (n., v.). To give an approximate |
| value close to an actual value; an | |
| imprecise calculation. | |
| eth- | (prefix). Containing two carbons. |
| eutrophication | (n). Excess nutrients in water |
| causing excessive plant growth and | |
| strangulation of a waterway. | |
| evaporate | (v). To change phase of matter |
| from liquid to gas. Compare | |
| sublimate and boil. | |
| excited | (n). The state of being in a higher |
| energy level (higher than ground | |
| state). | |
| exo- | (prefix). Outside of. |
| exothermic | (adj). Giving off heat, ΔH < 0. See |
| enthalpy, endothermic. | |
| exponent | (n). When a number is raised to a |
| power, i.e. multiplied by itself as | |
| many times as shown in the power | |
| (the small number up above the | |
| base number). So, 23 means | |
| 2 × 2 × 2. See also cubed. | |
| exponential | (adj). To multiply something many |
| times; a curve representing an | |
| exponent. |
| extrapolation | (n). To extend the line of a graph | ||
| further, into values not empirically | |||
| documented, to project a future | |||
| event or result. In plain language: | |||
| to say what is going to happen | |||
| based on past results which were | |||
| obtained (gotten) by experiment | |||
| and measurement. If you have | |||
| a graph and have documented | |||
| certain results (e.g. change vs | |||
| time), and you draw the line further | |||
| in the same curve, to say what | |||
| future results you will get, that is | |||
| called ‘extrapolation’. See predict. | |||
| extrude, | (v., n.). To push something through | ||
| extrusion | a mould or shape, usually a liquid | ||
| or gel through a hole, to create a | |||
| new shape. | |||
| F | |||
| fahrenheit | (n). A temperature scale based | ||
| on human body temperature. | |||
| Water freezes at 32°F and boils | |||
| at 212°F under standard | |||
| conditions. The conversion | |||
| formula to centigrade/celcius is: | |||
| (˚F – 32) × 5/ | 9 | = ˚C | |
| favoured | (adj). Preferred. Chemistry: in a | ||
| chemical reaction, the direction of | |||
| the reaction after the equilibrium | |||
| is broken. | |||
| fermentation | (n). The conversion of a sugar or | ||
| carbohydrate to an alcohol, usually | |||
| by yeast or bacteria. | |||
| fertilisation | (n). The process of adding | ||
| nutrients to soil. Biology: the fusion | |||
| of male and female gametes (sex | |||
| cells) into a zygote (group of cells | |||
| that will become a foetus or other | |||
| living organism). | |||
| fertiliser | (n). A substance added to soil for | ||
| nutritional reasons. | |||
| fixation | (n). The process of fixing on | ||
| something or someone. | |||
| fixed | (n). The process of attaching one | ||
| (chemistry) | chemical or compound to another. | ||
| E.g. nitrogen fixing is the bonding | |||
| of free N2 to organic chemicals. | |||
| fluid | (n). Any substance that can flow | ||
| and take the shape of a container; | |||
| liquid, some gels, and gas. | |||
| fluorescent | (n). Bright, colourful, due to | ||
| changes in energy levels of | |||
| electrons, as seen in fluorescent | |||
| tubes, ink, etc. See reflective. | |||
| formic | (prefix). Same as meth-. Contains |
| one carbon. From Latin “formica”: | |
| ant, referring to formic acid, which | |
| is found in an ant’s sting. | |
| fraction | (n). Mathematics: Not a whole |
| number; a representation of a | |
| division. A part. E.g. the third | |
| fraction of two is 0,666 or ⅔. | |
| meaning two divided into three | |
| parts. Chemistry: a part of a | |
| solution or mixture separated out | |
| by distillation. See distil. | |
| function | (n). Mathematics: when two |
| attributes or quantities correlate. | |
| If y changes as x changes, then | |
| y = f(x). See correlate, graph, | |
| Cartesian, axis, coordinate. Also: | |
| a relation with more than one | |
| variable (mathematics). Chemistry: | |
| functional group: part of a | |
| molecule that gives the substance | |
| its chemical properties in common | |
| with other similar chemicals. | |
| G | |
| galvanic | (adj). Relating to currents caused |
| by a chemical reaction. See | |
| electrochemistry. | |
| galvanising | (n). To electroplate so as to protect, |
| e.g. cover iron with zinc to prevent | |
| rust. | |
| gas | (n). The third phase of matter. |
| When a solid is heated it turns into | |
| liquid, and when a liquid is heated | |
| it turns into gas. | |
| gaseous | (adj). In a gas form. |
| gradient | (n). A slope. An increase or |
| decrease in a property or | |
| measurement. Also the rate of such | |
| a change. In the formula for a line | |
| graph, y = mx + c, m is the gradient. | |
| gradually | (adv). To change or move slowly. |
| graph | (n). A diagram representing |
| experimental or mathematical | |
| values or results. See Cartesian. | |
| graphic | (n., adj.). A diagram or graph |
| (n). Popular use: vivid or clear or | |
| remarkable (adj.). | |
| graphically | (adv). Using a diagram or graph. |
| Popular use: to explain very clearly. | |
| groundwater | (n). Water held in the earth |
| (underground). | |
| gypsum | (n). Calcium sulphate. |
| H | |
| Haber (process) | (n). An industrial process to produce |
| ammonia from nitrogen and | |
| hydrogen, using an iron catalyst at | |
| high temperature and pressure. | |
| haemoglobin | (n). A compound containing |
| iron, found in red blood cells, | |
| responsible for carrying oxygen. | |
| half-cell | (n). One of the sides of an |
| electrochemical cell; one of the | |
| electrodes and the chemical | |
| solution around it. | |
| half-reaction | (n). The equation for the chemical |
| reaction occurring in a half-cell. | |
| halide | (n). A compound containing a single |
| halogen, e.g. NaCℓ. See halogen. | |
| halo- | (prefix). Containing a halogen. See |
| halogen. | |
| haloalkane | (n). An alkane bonded to a |
| halogen. See halogen. | |
| halogen | (n). Any of the elements fluorine |
| (F), chlorine (Cℓ), bromine (Br), | |
| iodine (I), and astatine (At), in | |
| group VIIA (17) of the periodic | |
| table. They combine with metals to | |
| produce salts. See salt. | |
| halogenation | (n). Adding a halogen. |
| hardness | (n). (Water). Containing salts, usually |
| calcium carbonate. If water contains | |
| too many such salts, soap does | |
| not function properly and doesn’t | |
| produce bubbles or foam. “Water’s | |
| hardness is determined by the | |
| concentration of multivalent cations | |
| in the water. Multivalent cations are | |
| cations (positively charged metal | |
| complexes) with a charge greater | |
| than 1+. Usually, the cations have | |
| the charge of 2+. Common cations | |
| found in hard water include Ca2+ | |
| and Mg2+.” (Wikipedia). | |
| heat | (n). Physics: a measure of the |
| average kinetic energy of the | |
| molecules or atoms in a substance; | |
| enthalpy; the energy of an object | |
| as molecular motion. Alternatively, | |
| infra-red radiation (heat radiation) | |
| coming off a body. See body. | |
| homologous | (n). Belonging to the same group |
| of things; analogous. Biology: | |
| a flipper is homologous with a | |
| leg or arm. Chemistry (organic): | |
| belonging to the same series of | |
| molecules, e.g. alkanes: methane, | |
| ethane, propane; having the same | |
| functional group. |
| hydrate | (n). To add water to. | |
| hydration | (n). Having had water added. | |
| Some salts are hydrated, meaning | ||
| that they have a number of water | ||
| molecules bonded to them, which | ||
| can be removed by heat. Heating | ||
| a hydrated salt changes its colour | ||
| but not the chemical reactions it | ||
| will undergo. See salt. | ||
| hydrocarbon | (n). Any compound consisting | |
| mainly of hydrogen and carbon. | ||
| Compare carbohydrate. | ||
| hydrochloric | (adj). Any chemical containing HCℓ. | |
| hydrohalogen- | (n). Adding a hydrogen and halogen | |
| ation | atom to a molecule. | |
| hydrolysis | (n). Splitting by reacting with | |
| water. Applies to salts and organic | ||
| chemicals. E.g. A haloalkane plus | ||
| water or a dilute NaOH gives an | ||
| alcohol and either a hydrohalide | ||
| or sodium salt. Dissolving a | ||
| salt in water can be considered | ||
| hydrolysis. | ||
| hydronium | (n). H | O+ ion. |
| 3 | ||
| hydroxide | (n). OH– ion. This usage applies | |
| specifically to bases (see acid, | ||
| base). In organic molecules, OH– is | ||
| an alcohol functional group called | ||
| the hydroxyl group. See alcohol, | ||
| hydroxyl, diol. | ||
| hydroxyl | (n). See hydroxide. | |
| hydroxylamine | (n). An amine with a hydroxyl | |
| group. | ||
| I | ||
| ideal | (adj). Not as seen in real | |
| life; theoretical. Ideal gas: a | ||
| hypothetical gas whose molecules | ||
| occupy negligible space and | ||
| have no interactions, and which | ||
| consequently obeys the gas laws | ||
| (PV = nRT) exactly. | ||
| ignition | (n). The start of a combustion | |
| reaction. Common use: to start | ||
| a car (which has an internal | ||
| combustion engine). See engine, | ||
| combustion. | ||
| illuminate | (v). To explain or light up. | |
| immerse | (v). To cover in liquid. | |
| impair | (v). Prevent; hinder; slow down. | |
| impure | (adj). Containing a variety of | |
| additional chemicals in smaller | ||
| amounts in addition to the main | ||
| chemical. | ||
| incandescent | (adj). Giving off light as a result of |
| being heated. | |
| independent | (n). The things that act as input |
| (variable) | to the experiment, the potential |
| causes. Also called the controlled | |
| variable. The independent variable | |
| is not changed by other factors, and | |
| we plot it on the horizontal axis. See | |
| control, dependent variable. | |
| indicator | (n). Chemistry: a substance used |
| to check for pH levels, which | |
| changes colour according to pH. | |
| See acid, base, pH. | |
| indigo | (n). The colour between violet and |
| blue; purplish-blue. | |
| inert | (adj). Chemistry: a chemical or |
| element which does not react or is | |
| difficult to cause to react, e.g. Ne, | |
| Xe, He. N2 is sometimes described | |
| as inert but it’s not in the group of | |
| Noble Gases. Common use: lazy, | |
| unwilling to move. | |
| inflammable | (adj). Same as flammable; easily |
| set on fire (combustion). | |
| inhibitor | (n). Something that slows down or |
| prevents. | |
| inorganic | (adj). Not containing carbon; |
| mineral. Exceptions are C, CO, CO2, | |
| which, whilst they contain carbon, | |
| are not considered organic as they | |
| can be produced during inorganic | |
| chemical reactions. | |
| insoluble | (adj). Not able to dissolve. |
| insufficient | (adj). Not enough. |
| interact | (v). To affect each other, to be |
| directly involved with or act on | |
| each other. | |
| intermediate | (adj). A state in between. |
| intermolecular | (adj). Between molecules. See |
| molecule, intramolecular. | |
| intramolecular | (adj). Within or inside a molecule. |
| See molecule, intermolecular. | |
| inversion | (n). Chemistry: turning something |
| upside down. | |
| ion | (n). An atom or molecule or part of |
| a molecule which has an electrical | |
| charge due to gaining or losing one | |
| or more electrons. | |
| ionic (bond) | (adj.). A bond in which electrons |
| have been transferred from one | |
| side of the molecule to another | |
| resulting in a cation and anion, | |
| which then attract. E.g. NaCℓ. | |
| ionisation | (n). The process of ionising. See |
| ionise. | |
| ionise | (v). To turn into an ion. See ion. |
| irreversible | (adj). Cannot be reversed. Said |
| of certain chemical reactions, | |
| in which case it specifically | |
| means that the reaction does not | |
| spontaneously reverse (not an | |
| equilibrium reaction). The reaction | |
| only proceeds in one direction. | |
| Example: combustion. | |
| isolate | (v). To separate from. Usual use |
| in Physical Science means to | |
| separate one chemical from | |
| another. Compare insulate, distil. | |
| isomer | (n). A substance with the same |
| empirical formula but a different | |
| structural formula. See empirical, | |
| structural. | |
| isotope | (n). An element which has a |
| different number of neutrons from | |
| the usual number of neutrons in the | |
| element. E.g. 12C has 6 protons and | |
| 6 neutrons, but 14C has 8 neutrons | |
| and 6 protons, and is radioactive. | |
| IUPAC | (abbr). International Union of Pure |
| and Applied Chemistry. Standardised | |
| naming conventions for chemicals. | |
| J | |
| joule | (n). Unit of energy. |
| K | |
| kelvin | (n). Unit of temperature, with |
| absolute zero being the point where | |
| no molecular motion occurs, at | |
| -273,15˚C. Hence, the freezing point | |
| of water is 273,15 K. Note that there | |
| is no degree sign before K. | |
| ketone | (n). An organic compound with the |
| carbonyl group = C = O. Made by | |
| oxidising secondary alcohols. E.g. | |
| acetone. | |
| L | |
| law | (n). In Physical Science, a |
| formula or statement, deduced | |
| (discovered) from observation | |
| (watching). The formula or | |
| statement will then predict that | |
| under the same conditions the | |
| same thing will always happen. E.g. | |
| the first law of thermodynamics | |
| says that matter and energy | |
| cannot be destroyed, but only | |
| changed from one form to another. |
| leach | (v). When a substance drains out |
| of soil, e.g. into rivers. Similar to | |
| “leak”. | |
| litmus | (n). A type of acid/base indicator. |
| See indicator. It is red when | |
| exposed to acid and blue when | |
| exposed to a base. | |
| M | |
| macromolecule | (n). A large molecule, usually a |
| polymer or protein. | |
| macroscopic | (adj). Large enough to be visible |
| to the unaided human eye; big | |
| enough to be seen. | |
| magenta | (n). A bright purple/pink colour. |
| manipulate | (v). To change, or rearrange |
| something. Usually in Mathematics | |
| it means to rearrange a formula to | |
| solve for (to get) an answer. | |
| material | (n). Any substance, not just cloth. |
| matter | (n). Substance; stuff. Opposite of |
| vacuum (nothing). | |
| mean | (n). See average. |
| median | (n). Mathematics: the number in |
| the middle of a range of numbers | |
| written out in a line or sequence. | |
| metal | (n). A substance which is malleable |
| (can be hammered flat), is ductile | |
| (can be drawn into a wire), which | |
| conducts electricity and heat well | |
| and which is reflective (most light | |
| striking it is emitted again). Most | |
| elements are metals except the | |
| few on the right hand side of the | |
| periodic table starting at Boron (B) | |
| and running diagonally down to | |
| Astatine (At). | |
| meth- | (prefix). Having one carbon. See |
| formic. | |
| methylated | (adj). Having had a single carbon |
| or methyl group added. | |
| metric | (adj). A measurement system, |
| using a base of 10 (i.e. all the | |
| units are divisible by 10). The USA | |
| uses something known as the | |
| Imperial system, which is not used | |
| in science. The Imperial system is | |
| based on 12. Examples: 2,54 cm | |
| (metric) = 1 inch (imperial). | |
| 1 foot = 12 inches = approx. | |
| 30 cm; 1 metre = 100 cm. 1 Fl.Oz | |
| (fluid ounce) = approx 30 mℓ. | |
| microscopic | (adj). Too small to be seen by the |
| unaided human eye. |
| minimum | (n). The smallest amount possible. |
| mixture | (n). When you mix or combine |
| substances without them | |
| undergoing a chemical reaction. In | |
| other words, the substances mixed | |
| stay separate (chemically) and do | |
| not bond. Different to compound. | |
| See compound, reaction. | |
| modal | (adj). Pertaining to the mode, or |
| method. Can mean: about the | |
| mathematical mode or about the | |
| method used. See mode. | |
| mode | (n). Mathematics: the most |
| common number in a series of | |
| numbers. See also mean, median. | |
| mol | (abbr). Mole. |
| molar | (adj). About a mole. See mole. |
| mole | (n). A unit describing an amount |
| of substance. 6,023 × 1023 | |
| molecules or atoms of the | |
| substance. | |
| E.g. 18 g of water is 1 mol of | |
| water (H = 1, O = 16, H2O = 18). | |
| molecular | (adj). About molecules. See |
| molecule. | |
| molecule | (n). The smallest amount of a |
| compound; a single particle | |
| composed of the elements that | |
| make up the compound. E.g. in | |
| water, a single particle consisting | |
| of two hydrogen atoms and one | |
| oxygen atom. | |
| mono- | (prefix). One. |
| monomer | (n). Part of a macromolecule; the |
| simplest repeating unit. Monomers | |
| bond to form polymers. See | |
| polymer, isomer. | |
| monoprotic | (adj). Having one proton. |
| N | |
| neutral | (adj). Chemistry: pH 7,0. Neither |
| acid nor base. E.g. water. In | |
| common use: not biased. See bias. | |
| neutralise | (v). To make something neutral; to |
| complete an acid/base titration. | |
| neutron | (n). A subatomic particle with |
| no charge, mass approximately | |
| the same as a proton, found in the | |
| nucleus of an atom. Symbol n0. | |
| If there are too many protons in | |
| a nucleus, the substance will be | |
| radioactive as it releases | |
| alpha particles (helium nuclei, | |
| 2p+ + 2n0). |
| nitrate | (n). Containing NO −. |
| 3 | |
| nitrite | (n). Containing NO −. |
| 2 | |
| nomenclature | (n). A system of describing things; |
| a naming system, designed to | |
| make a name unambiguous or | |
| unique. | |
| nonmetal | (n). Any of the elements that are |
| not metals, e.g. Boron (B), Silicon | |
| (Si), Sulphur (S), Oxygen (O), etc. | |
| nucleus (sing), | (n).The centre of something |
| nuclei (pl), | (generally), specifically the centre |
| nuclear (adj) | of an atom, consisting of at least |
| one proton (hydrogen), or two | |
| protons and two neutrons (helium). | |
| Plural nuclei is pronounced “noo- | |
| klee-eye”. | |
| numerator | (n). The opposite of a denominator; |
| the number on top in a fraction. | |
| O | |
| -oate | (suffix). An ester. |
| odour | (n). A smell. |
| optimal | (adj). Best, most. |
| organic | (adj). Containing carbon, except C, |
| CO, CO2. | |
| origin | (n). Mathematics: the centre of |
| a Cartesian coordinate system. | |
| General use: the source of | |
| anything, where it comes from. | |
| outlier | (n). Statistics: a data point which |
| lies well outside the range of | |
| related or nearby data points. | |
| oxalic (acid) | (n). Ethanedioic acid; chem. |
| formula: (COOH)2. | |
| oxidation | (n). Specifically, adding an oxygen |
| atom to a molecule, but more | |
| general use: losing electrons from | |
| any substance in a redox reaction. | |
| See redox. | |
| oxide | (n). A compound containing |
| an oxygen atom, especially if it | |
| previously did not contain one, e.g. | |
| iron (metal), vs iron oxide (rust). | |
| oxidise | (v). To add an oxygen or remove |
| electrons from a substance. | |
| P | |
| paraffin | (n). Any waxy organic substance, |
| previously the general name for | |
| alkanes. Formula ranges from | |
| C20H42 to C40H82. |
| parallel | (adj). Keeping an equal distance |
| along a length to another item | |
| (line, object, figure). Mathematics: | |
| two lines running alongside each | |
| other which always keep an equal | |
| distance between them. | |
| particle | (n). Any small part, e.g. a proton, |
| an atom, a molecule. | |
| pascal | (n). The unit of pressure, |
| abbreviated Pa, units: N/m2 | |
| pent- | (prefix). Five. |
| per | (prep). For every, in accordance |
| with. Chemistry: the maximum | |
| amount of an element possible | |
| for the number of bonds available. | |
| See e.g. peroxide. | |
| peroxide | (n). H2O2. |
| perpendicular | (adj). Normal; at right angles to |
| (90˚). | |
| phase | (n). Time, period; a state of matter |
| (solid, liquid, gas); the relationship | |
| in time between the cycles of a | |
| system (such as an alternating | |
| electric current or a light or sound | |
| wave) and either a fixed reference | |
| point or the states or cycles of | |
| another system with which it | |
| may or may not be synchronised | |
| (simultaneous). I.e. if two systems | |
| vibrate at the same time at the | |
| same rate, they’re “in phase”. | |
| photosynthesis | (n). The process of converting |
| CO2 into carbohydrates using | |
| atmospheric CO2, chlorophyll, and | |
| light. | |
| pi | (n). π, the Greek letter p, the |
| ratio of the circumference of a | |
| circle to its diameter. A constant | |
| without units, value approximately | |
| 3,14159. | |
| plastic | (n., adj.). An artificial substance |
| made from hydrocarbon polymers | |
| which is often flexible and able to | |
| be moulded and is often a poor | |
| electrical conductor (n); flexible | |
| (adj). | |
| plot | (v). To place points on a Cartesian |
| coordinate system; to draw a | |
| graph. | |
| poly- | (prefix). Many. |
| polyester | (n). A polymer made from esters. |
| polymer | (n). A synthetic substance made |
| from many monomers (repeating | |
| units). See monomer. | |
| polymerisation | (n). Making a polymer. |
| polyprotic | (adj). Having many protons. |
| porous | (adj). Having many holes that allow |
| fluids through. | |
| positive | (adj). Having many protons not |
| paired with electrons; a lack of | |
| electrons. | |
| potential | (n). Having the ability to do work, in |
| particular, Ep (potential energy, the | |
| tendency to fall or start moving, | |
| as in a spring), or emf (voltage). | |
| General use: potential exists when | |
| there is an energy difference | |
| between two points, e.g. due to | |
| gravity or electrical charge. In the | |
| context of electricity, read it as | |
| “voltage”. | |
| precipitate | (n). Chemistry: A product of a |
| reaction that cannot dissolve in the | |
| solution and settles at the bottom | |
| of the reaction vessel (container). | |
| predict | (v). General use: to foresee. |
| Physical Science: to state what will | |
| happen, based on a law. See law. | |
| pressure | (n). A continuous force exerted on |
| an object over a certain area, in | |
| pascals, Pa. N/m2. See pascal. | |
| product | (n). Chemistry: the substance or |
| compound made as a result of a | |
| chemical reaction. See reaction. | |
| Mathematics: the result of | |
| multiplying two numbers. | |
| project | (n. & v.). A project (n., pronounced |
| PRODJ-ekt) is a plan of action | |
| or long-term activity intended to | |
| produce something or reach a goal. | |
| To project (v., pronounced prodj- | |
| EKT), is to throw something, or to | |
| guess or predict (a projection). To | |
| project a result means to predict a | |
| result. See extrapolate. | |
| prop- | (prefix). Three carbons. |
| Pronounced “prope” (rhymes with | |
| “rope”). | |
| proportion | (n). To relate to something else |
| in a regular way, to be a part of | |
| something in relation to its volume, | |
| size, etc; to change as something | |
| else changes. See correlate and | |
| respectively. | |
| protein | (n). A large, complex organic |
| molecule containing nitrogen, | |
| usually making up structural | |
| elements of living things (building | |
| blocks of cells, antibodies, etc). | |
| protolytic | (adj). Capable of removing a |
| proton; proton transfer. Compare | |
| acid. |
| proton | (n). The positively-charged particle |
| that forms the centre of an atomic | |
| nucleus, weighing 1 836 times as | |
| much as an electron, but having | |
| the same and opposite charge. | |
| Symbol p+. See also nucleus, | |
| neutron, electron. | |
| pump | (n). A machine that uses energy |
| to transfer a fluid from one | |
| place to another. In Biology one | |
| finds cellular pumps, which are | |
| biological machines for transferring | |
| ions and nutrients. | |
| pure | (adj). Containing only the |
| compound or element in question, | |
| without any other compounds or | |
| elements mixed in. See impure. | |
| purification | (n). The process of removing |
| impurities. See distil. | |
| Q | |
| qualitative | (adj). Relating to the quality |
| or properties of something. A | |
| qualitative analysis looks at | |
| changes in properties like colour, | |
| that can’t be put into numbers. | |
| Often contrasted with quantitative. | |
| quantitative | (adj). Relating to, or by comparison |
| to, quantities. Often contrasted | |
| with qualitative. A quantitative | |
| analysis is one in which you | |
| compare numbers, values and | |
| measurements. | |
| quantity | (n). Amount; how much. |
| R | |
| rancid | (n). Having an unpleasant smell due |
| to having started to ferment or rot, | |
| usually said of meat, oil or butter. | |
| random | (n). Unpredictable, having no cause |
| or no known cause. Done without | |
| planning. | |
| range | (n). Mathematics: the set of values |
| that can be supplied to a function. | |
| The set of possible y-values in a | |
| graph. See domain. | |
| rate | (n). How often per second (or per |
| any other time period). Physics: | |
| number of events per second; see | |
| frequency. | |
| ratio | (n). A fraction; how one number |
| relates to another number; exact | |
| proportion. If there are five women | |
| for every four men, the ratio of | |
| women to men is 5:4, written | |
| with a colon (:). This ratio can be | |
| represented as the fraction 5/4 or | |
| 1¼ or 1,25; or we can say that | |
| there are 25% more women than | |
| men. | |
| react | (v). Chemistry: when two or more |
| elements or compounds are | |
| brought into a mixture and form | |
| chemical bonds, creating new | |
| compounds. | |
| reactant | (n). A chemical before it bonds with |
| another chemical. See reagent. | |
| reaction | (n). Chemistry: The process |
| of reacting; a state in which | |
| chemicals react. See endothermic | |
| and exothermic. Physics (nuclear): | |
| When a nucleus of an atom breaks | |
| down and subsequently releases | |
| energy and/or bonds with another | |
| nucleus. In the first case, it is a | |
| fission (splitting) reaction, in the | |
| second case a fusion (joining) | |
| reaction. | |
| reactive | (adj). Tending to react easily. |
| reactivity | (n). How reactive a substance is |
| (unreactive or reactive). | |
| reagent | (n). A reactant when it is still in its |
| bottle or container, before being | |
| mixed. | |
| redox | (abbr). Chemistry: reduction- |
| oxidation reaction; a chemical | |
| reaction in which one substance | |
| is reduced (gains electrons), and | |
| another is oxidised (loses electrons). | |
| See reduce, oxidise, anode, cathode, | |
| electrode, electrochemistry. | |
| reduce | (v). To make smaller. Chemistry: to |
| gain electrons (negative charges). | |
| reflux | (n., v.). A substance that flows back |
| into its container after coming out. | |
| Chemistry: the process of boiling a | |
| liquid so that any vapour is liquefied | |
| and returned to the stock (source). | |
| refraction | (n). Bending light when it travels |
| from one medium (e.g. air) into | |
| another medium (e.g. water or | |
| glass). Changing the direction of | |
| propagation of any wave as a result | |
| of its travelling at different speeds at | |
| different points along the wave front. | |
| See Huygens’ principle, diffraction. |
| S | |
| salt | (n). In common usage, NaCℓ. |
| Chemistry: any compound formed | |
| from the reaction of an acid with | |
| a base, with the hydrogen of the | |
| acid replaced by a metal or other | |
| cation. A non-metal ion bonded to | |
| a metal ion. | |
| saturated | (adj). Organic chemistry: Having |
| no available bonds or only single | |
| bonds. Common use: cannot take | |
| any more, usually said of a cloth | |
| and liquid. | |
| SI | (abbr). Système International. The |
| international system of metric | |
| units used by scientists. See | |
| metric, IUPAC. | |
| simplify | (v). To make simpler. Mathematics: |
| to divide throughout by a common | |
| factor (number or algebraic letter) | |
| that will make the equation easier | |
| to read and calculate. | |
| slaked (lime) | (n). Calcium Hydroxide, Ca(OH)2. |
| solubility | (n). How easily something dissolves |
| (mixes into a liquid). | |
| soluble | (adj). See solubility. |
| solute | (n). The substance that you place |
| in a liquid (the solvent) so as to | |
| dissolve it. E.g. salt. See solvent. | |
| solution | (n). A mixture of a solute and a |
| solvent. A liquid which has had | |
| something dissolved in it (mixed). | |
| Mathematics: the step-by-step | |
| displaying of calculations to | |
| arrive at answers. Common use: | |
| the answer to a problem, in the | |
| sense of dissolving (removing) a | |
| problem. | |
| solvent | (n). The liquid that dissolves the |
| substance placed into it. E.g. water. | |
| spectator | (n). Chemistry: a compound or |
| chemical which does not get | |
| involved in a chemical reaction. | |
| spontaneous | (adj). Randomly, without |
| provocation or cause or prior | |
| planning. | |
| stable | (adj). Chemistry and Nuclear |
| Physics: not likely to break down or | |
| react further. | |
| standardised | (adj). Chemistry: a solution of |
| known concentration, e.g. 1 molar | |
| (1 mol/dm3). |
| steam | (n). Water vapour, microscopic |
| droplets of water. Not a gas, a | |
| suspension of water droplets in air. | |
| See suspension, gas, liquid, phase, | |
| aerosol. | |
| STP | (abbr). Standard temperature and |
| pressure; 101,3 kPa and 25˚C. | |
| structural | (adj.). Pertaining to structure; |
| (isomer) | a series of molecules whose |
| structures are different but their | |
| chemical or empirical formulae are | |
| the same. | |
| sublimate | (v). To change phase of matter |
| from solid straight to gas without | |
| the intermediate phase of liquid. | |
| See the case of dry ice (CO2). | |
| subscript | (n). A number placed below the |
| rest of the line, e.g. CO2. | |
| substance | (n). Matter. Physical things. |
| substituent | (n). Chemistry: an ion or functional |
| group or group of atoms that | |
| replaces a hydrogen on an organic | |
| molecule. | |
| substitute | (v). To replace. |
| substitutents | (n). Something that gets replaced. |
| substitution | (n). The process of substituting. |
| Mathematics: to replace an | |
| algebraic symbol in a formula with | |
| a known value or another formula, | |
| so as to simplify the calculation. | |
| See simplify. Chemistry: to | |
| cause a substituent to bond to a | |
| substance. | |
| superscript | (n). A number placed above the |
| rest of the line, e.g. πr2. | |
| synthesis | (n). The process of |
| manufacturing (making) | |
| something. Chemistry: to bond | |
| smaller molecules together | |
| to create a larger molecule, | |
| e.g. methanol from CO and H . | |
| Synthesis gas: a gas mixture 2 | |
| (e.g. CO, H2), which when heated | |
| produces a new compound, e.g. | |
| methanol. | |
| synthetic | (adj). Artificial, man-made. |
| system | (n). Any closely associated and |
| inter-related or inter-dependent | |
| group of things; a set of things | |
| working together. Chemistry: a | |
| vessel (container) which contains a | |
| chemical reaction. |
| T | ||
| terminal | (n). Final; end point. | |
| termination | (n). Coming to an end. | |
| tetra- | (prefix). Four. | |
| theory | (n). A usually mathematical | |
| representation of an explanation | ||
| for something in the sciences, | ||
| which does not depend on the | ||
| thing being explained. A theory | ||
| differs from a law in that theories | ||
| are prone to empirical (visible | ||
| or measurable) refutation | ||
| (rejection); meaning that they | ||
| can be discarded if evidence | ||
| comes in that they are wrong. | ||
| See law. | ||
| thermoplastic | (n). Chemistry: will melt if heated. | |
| thermoset | (n). Once set into a shape it cannot | |
| melt again. | ||
| thiosulphate | (n). A salt containing the anion | |
| S | O 2−. | |
| 2 | 3 | |
| threshold | (n). Physical Science: the | |
| magnitude or limit of something, | ||
| which, if exceeded, will cause | ||
| something else, e.g. release of | ||
| radiation, a chemical reaction, etc; | ||
| the minimum amount of energy | ||
| required to cause something. | ||
| Medicine: the maximum safety | ||
| level of a dose. | ||
| titrate | (v). To measure off a reagent | |
| precisely drop by drop into a vessel | ||
| (container) containing another | ||
| reagent, so as to work out the | ||
| concentration of the reagent in the | ||
| vessel. | ||
| toxic, toxin | (adj., n.). Poisonous, poison. | |
| transfer | (n). To move from one place to | |
| another. Chemistry: usually refers | ||
| to moving an electron from one | ||
| compound to another. Finance: | ||
| usually refers to a payment | ||
| or credit. See credit, debit, | ||
| transaction. | ||
| trends | (n). Mathematics: regular patterns | |
| within data. | ||
| tri- | (prefix). Three. | |
| trial | (n). Chemistry: to repeat an | |
| experiment, an iteration, | ||
| or particular attempt at an | ||
| experiment. (From “try”, to try | ||
| once). | ||
| triprotic | (n). Having three protons. |
| turbidity | (n). How muddy, muddled or |
| opaque or disturbed a liquid is. | |
| U | |
| unit | (n). A subdivision of a scale. See |
| scale. | |
| universal | (n). A chemical which can indicate |
| (indicator) | how acid or basic a solution is, |
| ranging from reds (acidic) to violets | |
| (basic), including most of the | |
| colour spectrum. | |
| unsaturated | (n). Organic chemistry: having |
| double or triple bonds present. | |
| unstable | (n). Chemistry or Nuclear Physics: |
| prone to disintegrating or reacting. | |
| urea | (n). CO(NH2)2. The substance used |
| to remove excess nitrogen from | |
| animals via urination. Useful as a | |
| fertiliser. | |
| V | |
| vapour | (n). The pressure above a liquid |
| pressure | caused by molecules evaporating |
| from the surface of its liquid form, | |
| when in phase equilibrium (i.e. as | |
| many molecules leaving the liquid | |
| surface are condensing back into | |
| the liquid). | |
| variable | (n., adj.). A letter used to represent |
| an unknown quantity in algebra | |
| (n); a quantity that changes (n); | |
| subject to change (adj). |
| vessel | (n). Any container. Common use: a |
| container or ship. | |
| visible | (adj). Able to be seen by the human |
| eye, opposite of invisible. Compare | |
| microscopic, macroscopic. | |
| viscosity | (n). The thickness of a fluid. A |
| viscous fluid flows slowly, e.g. | |
| syrup. Pronounced “viss-KOSS- | |
| itee” and “viss-k’s”. | |
| volatility | (n). How easily something |
| evaporates. E.g. Ether (C2H5OC) is | |
| more volatile than water. | |
| volt | (n). Unit of potential difference |
| in electricity. The difference of | |
| potential (Ep) that would carry one | |
| ampere of current against one | |
| ohm resistance. Same as emf. See | |
| emf, resistance, ohm, ampere. | |
| voltage | (n). The measurement of volts. |
| voltaic | (n). The production of electricity |
| in a cell. See battery, cell, | |
| electrochemistry, electrode, | |
| cathode, anode, galvanic. | |
| volume | (n). A measure of the space |
| occupied by an object, equal to | |
| length x breadth x height. | |
| Y | |
| yield | (n., v.). The amount of substance |
| produced in a chemical reaction | |
| (n); products of a process (n); to | |
| hand over or give up (v). |
The maths you need
This section gives you the basic mathematical skills that you need to pass any subject that makes use of mathematics. Whether you’re studying for Physical Science, Mathematics, or Mathematical Literacy, these basic skills are crucial. Do not go any further in this book until you have mastered this section.
1. Basic pointers
- If a formula does not have a multiplication (×) sign or a dot-product (·), and yet two symbols are next to each other, it means “times”. So, m1m2 means mass 1 times mass 2. You can also write it as m1 × m2, or m1·m2
- Comma means the same as decimal point on your calculator (i.e. 4,5 = 4.5). Do not confuse the decimal point with dot product (multiply): 4.5 = 412 but 4·5 = 20. Rather avoid using the dot product for this reason.
- In science it is common to write divisors with an exponent. This means, for example, that 0,5 metres per second is usually written 0,5 m·s–1 rather than 0,5 m/s. Both notations are perfectly correct, however, and you may use either. It is important, however, that you either use –1 or / . If you just put 0,5 ms, that means 0,5 milliseconds, which is not a velocity (speed in a direction); it is a time. A variable is something that varies (means: changes). So, for example, the weather is a variable in deciding whether to go to the Variables in science and mathematics are represented with letters, sometimes called algebraic variables. The most common you see in maths is x, probably followed by y, z. In science, variables are given their letter symbols specifically depending on what they stand for; so, for example, M or m are used for mass (amount of substance in kilograms); v is used for velocity (speed in a certain direction); a is used for acceleration (change in velocity), etc. You can guess for the most part what a variable is for by what its letter is; so V is voltage, R is resistance, P is pressure, and so on.
2. Subject of formula or solving for
Very often you have to “make something the subject of a formula” or “solve for something”. This refers to finding the value of an unknown quantity if you have been given other quantities and a formula that shows the relationship between them.
Worked example 1
If John has 5 apples, and he gives some to Joanna, and he has two apples left, how many did he give to Joanna? Well, the formula would be something like this:
- 5 – x = 2
To solve for x, we simply have to swap the x and the 2. What we’re actually doing is adding “x” to both sides:
- 5 – x + x = 2 + x
this becomes:
- 5 = 2 + x
then we subtract 2 from both sides to move the 2 over:
- 5 – 2 = 2 – 2 + x
5 – 2 = x
3 = x … so John gave Joanna three apples.
The same procedures apply no matter how complex the formula looks. Just either add, subtract, square, square root, multiply, or divide throughout to move the items around.
Worked example 2
Let’s take an actual example from Electricity: V = IR. This means, the voltage in a circuit is equal to the current in the circuit times the resistance.
Suppose we know the voltage is 12 V, and the resistance is 3 Ω. What is the current?
- V=IR
12=3 × I
divide throughout by 3 so as to isolate the I
- 12 = (30)I
3 ( 3 )
remember that anything divided by itself is 1, so:
- 12 = (1) × I … and 12 = 4 …so
3 3
4 = I or
I = 4 A … The circuit has a current of 4 amperes.
It is possible to remember how to solve for these equations using a triangle mnemonic as follows:
If you’re solving for V, cover V with your hand. Then, I next to R means I times R, or IR. So, V = IR. If you’re solving for R, cover R with your hand. V is over I. So R = VI . While this is an easier way to do it, remember that many formulas do not consist of only three parts, so it is better to know how to make something the subject of a formula, or solve for something.

Worked example 3
Here’s a more tricky example. Given
- Kc = 4,5
[SO3] = 1,5 mol/dm3
[SO2] = 0,5 mol/dm3
[O2] = (X – 48) mol/dm3
Solve for x:
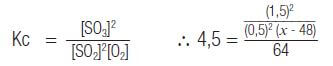
∴x = 176 g
How did we get that answer?
Step by step
Let’s see how it works.First, solve for the exponents (powers):

Now we multiply both sides by 576 to remove the 576 from the bottom row
- 576 = (x – 48) 576
4,5 576
and we cancel the 576’s on the right hand side as shown above.
Now, if 576 ÷ 4,5 = 128, then
- 128 = x – 48
Now we add 48 to both sides to move the 48 across
- 128 + 48 = x – 48 + 48 … hence, 128 + 48 = x = 176.
3. Statistics
Many experiments in science use statistics. You should therefore at least know the following:
Dependent variable: The thing that comes out of an experiment, the effect; the results.
Independent variable(s): The things that act as input to the experiment, the potential causes. Also called the controlled variable.
Control variable: A variable that is held constant in order to discover the relationship between two other variables. “Control variable” must not be confused with “controlled variable”.
It is important to understand that in science, correlation does not mean causation. That is, if two variables seem to relate to each other (they seem to co-relate), it doesn’t mean that one causes the other. A variable only causes another variable if one of the variables is a function f(x) of the other. We will see more about this when we look at graphs, below.
- Mean: The average. In the series 1, 3, 5, 7, 9, the mean is 1 + 3 + 5 + 7 + 9 divided by 5, since there are 5 bits of data. The mean in this case is 5.
- Median: The datum (single bit of data) in the precise middle of a range of data. In the series 1, 3, 5, 7, 9, the median value is 5.
- Mode: The most common piece of data. In the series 1, 1, 2, 2, 3, 3, 3, 4, 5, the mode is 3.
Often in scientific formulas it is said that things are proportional to each other. However, we cannot calculate the value of a force or energy output or mass etc., if we only know what things are in proportion (i.e. which things correlate).
Let’s take momentum for example. Momentum (how forcefully something moves, more or less), is proportional to velocity (speed in a direction). So the faster something’s moving, the more momentum it has. But p (momentum) can’t be calculated if we only know velocity; we need to know mass as well. Why? Because momentum is also proportional to mass; the more massive something is, the more momentum it has. Thus, to get rid of the proportionality sign (∝), we have to come up with a formula. Many experiments in science serve to find out what the relationship is between two variables, i.e. if they’re merely correlated — proportional — or if they’re causally related. In the case of momentum, it’s easy, because there are no further variables: p = mv. However, in the case of gravity or electric or magnetic field strengths, it’s not that easy. In those cases, we have to introduce something called a “constant”. A constant is a fixed value that is always multiplied into an equation. Constants are often written k. However, some specific constants, such as in the Law of Gravity, have their own symbol, in this case, G. These constants are given in the tables later in this book.
4. Graphs
A lot of work in science involves interpreting graphs. You get graphs of motion, graphs of rates of chemical reactions, graphs of distance-relative strengths of force fields, and so on. Before you can understand these graphs, it’s probably best to start from scratch with Cartesian Coordinates. “Coordinates” are numbers that refer to the distance of a point along a line, or on a surface, or in space, from a central point called the “origin”. Graphs that you will use have only two dimensions (directions). The positions of points on these graphs are described using two coordinates: how far across (left-to-right) the point is, called the x-coordinate, and how far up-or-down on the page the point is, called the y-coordinate.
Worked example 4
Consider the following graph. It shows six points in a straight line.

The coordinates shown can be described using what are called “ordered pairs”. For example, the furthest point in this graph is 3 units across on the “x-axis” or horizontal line. Likewise, it is also 3 units up on the y-axis, or vertical (up and down) line. So, its coordinates are (3;3). The point just below the midpoint or “origin”, is one unit down of the x-axis, and one unit left of the y-axis. So its coordinates are (-1;-1). Note that anything to the left or below of the origin (the circle in the middle), takes a minus sign. In most cases in science, you’ll only have graphs showing positive axes (plural of axis, pronounced aks-eez), since most graphs are of time
This series of dots look like they’re related to each other, because they’re falling on a straight line. If you see a result like this in an experimental situation, it usually means that you can predict what the next dot will be, namely, (4;4). This kind of prediction is called “extrapolation”. If you carry out the experiment, and find that the result is (4;4), and then (5;5), you’ve established that there is a strong relation or correlation. You can therefore start thinking about a formula to describe your findings. For example, this might be a graph showing a measurement of voltage (x) against a measurement of resistance (y).
Now, another way of saying that x relates to y, or x is proportional to y, is to say that y is a function of x. This is written y = f(x). So, in the example given above, voltage is a function of resistance. But how is y related to x in this graph? Well, it seems to be in a 1 to 1 ratio: y = x. So the formula for this graph is y = x. In this case, we’re only dealing with two factors; x and y. In other graphs you’ll find that sometimes more factors are involved, such as acceleration graphs, which have units of m/s2. Don’t worry about that; you treat them the same way (for example, m/s2 vs. time).
Worked example 5
Now, let’s take a slightly more complex case, illustrated next to this paragraph. In this graph, we see that wherever x is equal to something, y is one more. So, trace your finger from the bottom left dot upwards. It meets the x-axis at the point -3. Do the same for the same point towards the y-axis. You’ll see it meets the y-axis at -2. You’ll see the next coordinates are (–2;–1), then (–1;0), then (0;1), (1;2), and finally (2;3). From this we can see that whatever x is, y is one more. So, y = x + 1 is the formula for this line.

Worked example 6
Let’s take another case. In this next case, we see the following values: where x has a certain value, y has double that value. Let’s tabulate it.

| x | y |
| 1,5 | 3 |
| 1 | 2 |
| 0,5 | 1 |
| 0 | 0 |
| –0,5 | –1 |
| 1 | –2 |
| –1,5 | –3 |
So, when x is 1,5, y is 3, when x is 1, y is 2. Thus, the formula for this line is: y = 2x. This value next to x is called the “gradient” or “slope” of the line. The larger the value next to x is, i.e. the larger the gradient, the steeper the slope. The gradient is usually abbreviated as “m” when it is unknown.
Now, how this applies to science is simple: if we are looking, for example, at a case of a graph of a chemical reaction, we will usually have the x-axis as time. And the y-axis will usually be the quantity (amount) of substances produced. So, if we have a graph of a chemical reaction with a large gradient, it means that the reaction is fast; a lot of substance (y) i s produced in a short time (x). If, for example, we heated the reaction, and saw that the gradient increased even more, that would show that the chemical reaction was sped up by heat, or, that reaction rate is proportional to heat. Likewise, if the gradient sloped downwards, it would show that the reaction slowed down over time, because y, the amount of substance produced, was decreasing, as x (time) increased, e.g. because the reactants were being used up.
Worked example 7
Let’s do one more case. In this case, we can see that y is a function of x, since it’s a straight line graph. However, it’s not that easy to see the relationship between x and y. We can see that the slope is the same as the previous graph, so it must be something like y = 2x. However, it doesn’t quite make sense, since 2(–1,5) is not –2. We see that where x is zero (at the origin), y is at 1. But the slope is the same, so it must be y = 2(0) + 1. So the formula is: y = 2x + 1.

| x | y | 2x + 1 |
| -1,5 | -2 | 2(–1,5)+1 = –3+1 = –2 |
| -1 | -1 | 2(–1)+1 = –2+1 = –1 |
| -0,5 | 0 | 2(–0,5) +1 = –1+1 = 0 |
| 0 | 1 | 2(0)+1 = 0+1 = 1 |
| 0,5 | 2 | 2(0,5)+1 = 1+1 = 2 |
| 1 | 3 | 2(1)+1 = 2+1 = 3 |
Resource sheets
The following information sheets will be supplied to you in the exam. You do not need to memorise them.
SI Units: Multipliers
| Prefix | Symbol | Value | Value written in full |
| tera | T | 1012 | 1 000 000 000 000 |
| giga | G | 109 | 1 000 000 000 |
| mega | M | 106 | 1 000 000 |
| kilo | k | 103 | 1 000 |
| hecto | h | 102 | 1 00 |
| deka | da | 101 | 1 |
| deci | d | 10–1 | 0,1 |
| centi | c | 10–2 | 0,01 |
| milli | m | 10–3 | 0,00 1 |
| micro | µ | 10–6 | 0,00 000 1 |
| nano | n | 10–9 | 0,00 000 000 1 |
| pico | p | 10–12 | 0,00 000 000 000 1 |
| femto | f | 10–15 | 0,00 000 000 000 000 1 |
Constants
| Name | SI Unit Symbol | Approximate Value | Easier to Understand |
| STP (Standard Temperature and Pressure), (in Physics). | not applicable, two conditions | 1 ATM (101,3 kPa), 25°C (298 Kelvin (K)) | You generally put two ATM (bar) pressure in car tyres, i.e. the pressure in a car tyre is twice atmospheric pressure |
| Standard Conditions (Chemistry) | not applicable, three conditions | STP plus 1 mol/dm3 | As above |
| Gas constant | R | 8,3 J / mol⋅K | The R in PV = nRT |
| Molar gas volume at STP | V0 | 22,4 dm3 / mol | 22,4 Litres of gas is made by a mole of a substance |
| Avogadro’s constant | NA | 6,022045 × 1023 units/mol | 6 022 000 000 000 000 000 000 00 particles is one mole |

Formulas
Moles, Gas Laws, Chemical Equilibria:
= gas constant
= number of moles
m = mass
T= temperature
V= volume
c= concentration, also [ ]
- n = m c = n c = m
M V MV - PV = nRT P1V1 = P2V2
T1 T2

Where [S] is the concentration of S in mol/dm3
Notes:
In most cases in chemistry, subscripts refer to how many atoms there are; e.g. H2O = two atoms of H & 1 atom of O.
Standard reduction potentials

Notes:
- Eθ means the same as E0.
- There are two versions of this table; they are identical except one is upside-down. Just memorise that Fluorine (F) has the greatest oxidising ability.
- A strong reducing agent will displace a weaker reducing agent from its salt.
- Always start with the oxidation half reaction.
- A redox reaction will take place when a reducing agent reacts with an oxidising agent.
- Balance the electron charge of each half-reaction by multiplying each with a suitable coefficient.
- Add the two half-reactions together, eliminating electrons from both sides.
- Eliminate common ions or molecules from both sides of the equation e.g. H+ and H2
- You can then combine the E0 voltages to get the total voltage of a cell. Use the values exactly as they are; do not round off.
- E0cell = E0cathode – E0anode
OR - E0cell = E0oxidising agent – E0reducing agent
- E0cell = E0cathode – E0anode
- A positive answer means that the reaction will proceed spontaneously from left to right. A negative value means that it is not spontaneous.
